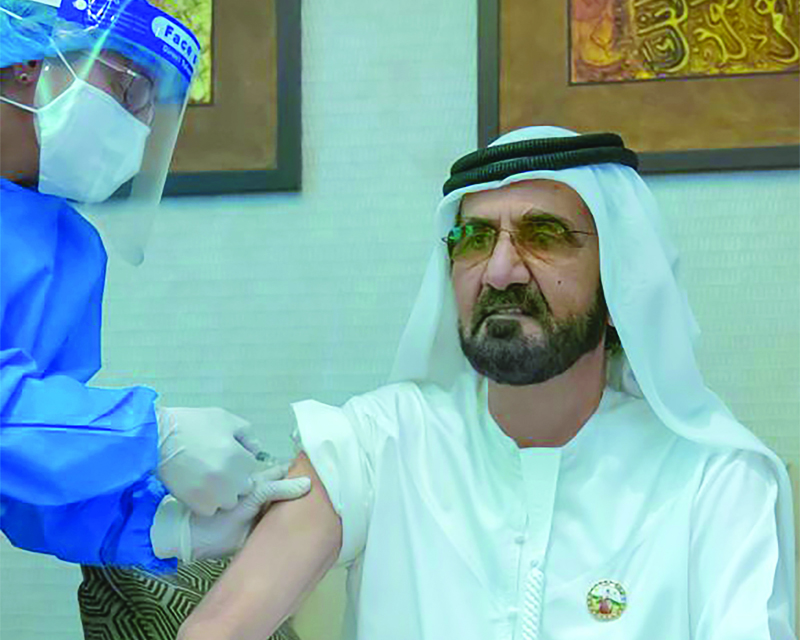
DUBAI: Dubai ruler Sheikh Mohammed bin Rashid Al-Maktoum said yesterday he had received an experimental coronavirus vaccine, becoming the latest United Arab Emirates official to take part in the trials. Two vaccines are undergoing third-phase trials in the UAE, one produced by Chinese drug giant Sinopharm, and Russias Sputnik-V, named after the Soviet-era satellite.
While receiving the COVID-19 vaccine today, Sheikh Mohammed captioned a photograph of himself he posted on Twitter with his sleeve rolled up, as a healthcare worker in full protective equipment administered the injection. We wish everyone safety and great health, and we are proud of our teams who have worked relentlessly to make the vaccine available in the UAE, he said, without specifying which vaccine he took.
Chinese drug giant Sinopharm began the third phase of trials for a COVID-19 vaccine in the UAE in July and Emirati officials say the results have been positive. Last month, authorities announced the UAE had also approved clinical trials of Russias Sputnik V, becoming the first Middle East country to do so. Sheikh Mohammed is also vice-president and prime minister of the UAE, a collection of seven emirates which has so far recorded more than 136,000 coronavirus cases, 503 of them fatal.
Meanwhile, Bahrain has granted emergency approval for the use of Sinopharms vaccine candidate currently in phase III trials on frontline workers from yesterday, state news agency BNA said. The vaccine candidate, nearing the end of phase III trials in the UAE, Egypt, Bahrain and Jordan, is a partnership between Sinopharms China National Biotec Group (CNBG) and Abu Dhabi-based artificial intelligence and cloud computing company Group 42 (G42).
The UAE in September authorized similar emergency use of the same vaccine for frontline workers at high risk of infection with the new coronavirus. Bahrains Health Minister Faeqa bint Saeed Al-Saleh said yesterday, in comments carried by BNA, that the use of the vaccine complies with the countrys regulations on exceptional licensing in emergency cases. The results of phase I and phase II clinical trials showed the vaccine is safe and effective, she said, adding that phase III trials were going smoothly and without serious side effects.
Around 7,770 people have so far volunteered in the Phase III trials in Bahrain and have received a second dose, the minister added. The phase III trials of the inactivated virus vaccine began in mid-July in the UAE, and were expanded to Bahrain, Egypt and Jordan. G42 Healthcare CEO Ashish Koshy last month said the vaccine had been administered to more than 31,000 people across those countries.
Several ministers and senior officials have already received the vaccine in both the UAE and Bahrain. UAE Foreign Minister Sheikh Abdullah bin Zayed Al-Nahyan and Deputy Prime Minister Sheikh Saif bin Zayed Al-Nahyan have taken the experimental vaccine.EIn September, Bahrain announced that Crown Prince Salman had taken part in third phase trials of the Sinopharm vaccine.
Dozens of companies, from biotech start-ups to Big Pharma, are racing to develop a safe and effective COVID-19 vaccine. The World Health Organization has identified 42 candidate vaccines at the stage of clinical trials. Ten of them are at the most advanced phase three stage, in which a vaccines effectiveness is tested on a large scale, generally tens of thousands of people across several continents. - Agencies
.jpg)
